Type 1 diabetes is a chronic condition that affects millions of people worldwide. According to the World Health Organization (WHO), it is estimated that approximately 10% of all diabetes cases are type 1.
In a significant development, the FDA has approved the first cellular therapy for type 1 diabetes on June 28. This marks a major advancement in treatment options for individuals with this condition.
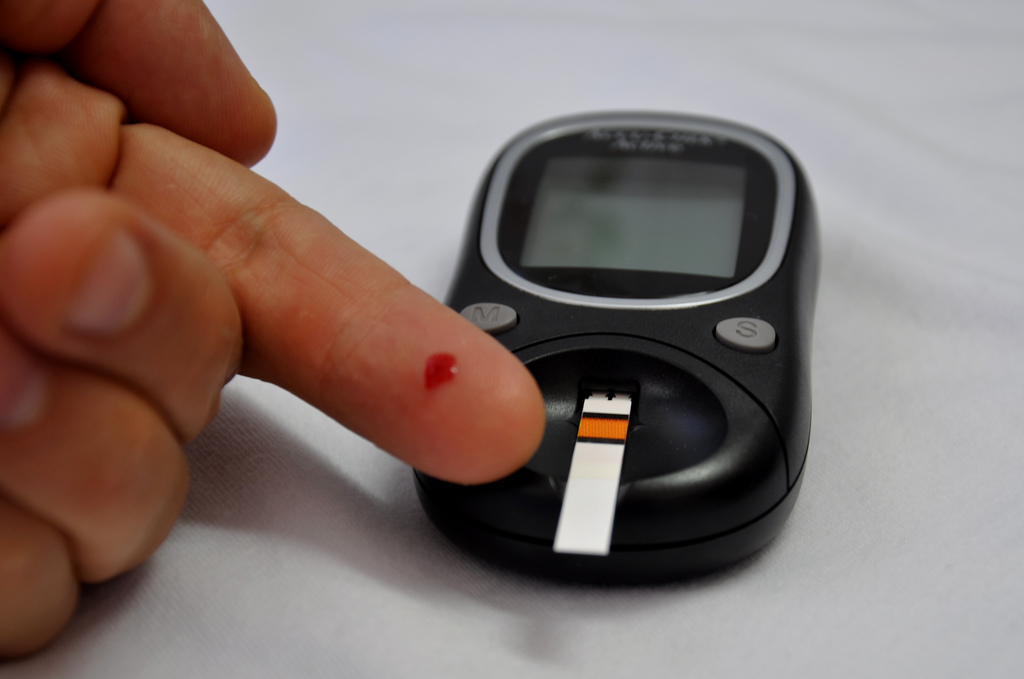
Type 1 diabetes is a chronic condition where the immune system attacks pancreatic cells that produce insulin, leading to insufficient insulin production. Insulin is crucial for regulating blood sugar levels by facilitating the movement of glucose from the blood into cells for energy. So, individuals with type 1 diabetes must monitor levels regularly and administer insulin as needed for stability.
The first approved treatment for individuals with type 1 diabetes
The drug, called Lantidra, is an infusion of insulin-producing pancreatic cells, sourced from deceased donors. It is the first approved treatment for individuals with type 1 diabetes who cannot regulate their blood sugar levels with standard insulin shots or infusions.
This approval is a major step forward. It provides a new treatment choice for individuals who have difficulty managing their blood sugar levels using regular insulin doses. These individuals often experience dangerously low blood sugar levels, a condition called hypoglycemia. Hypoglycemia is a dangerous condition that can cause loss of consciousness or seizures.