Hydrogen possesses immense potential in mitigating greenhouse gas emissions and addressing the impacts of climate change. Clean hydrogen has the potential to not only power vehicles without emitting pollutants but also play a crucial role in decarbonizing various industrial processes.
Worldwide researchers are actively pursuing cost-effective methods to produce clean hydrogen from water as a viable alternative to fossil fuels.
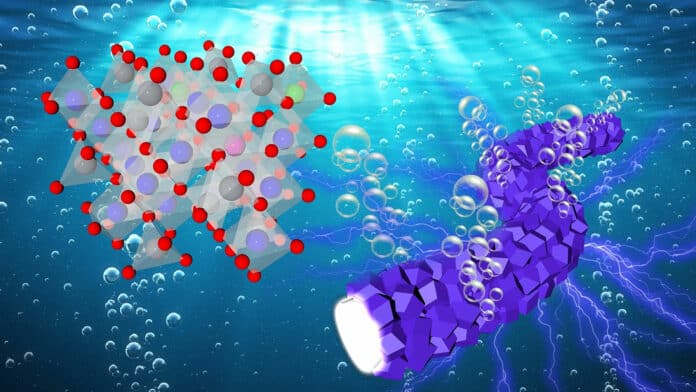
Water electrolysis is an environmentally sustainable method with a long history, dating back over a century, for producing hydrogen. However, the challenge lies in finding a stable and highly active oxidation catalyst that can efficiently operate in the acidic environment required for optimal performance. Currently, iridium is one of the rare and expensive catalysts that meet these requirements. The scarcity and high cost of iridium present significant obstacles to the widespread implementation of proton exchange membrane (PEM) electrolyzers.
New Low-cost Catalyst
A team of researchers, led by the U.S. Department of Energy’s Argonne National Laboratory, has made a significant breakthrough by developing a low-cost catalyst that enables the production of clean hydrogen from water. The key component of this catalyst is cobalt, which is considerably more affordable compared to iridium. This development holds promising implications for making hydrogen production more accessible and economically viable.
“We sought to develop a low-cost anode catalyst in a PEM electrolyzer that generates hydrogen at high throughput while consuming minimal energy,” said Di-Jia Liu, senior chemist at Argonne. “By using the cobalt-based catalyst prepared by our method, one could remove the main bottleneck of cost to producing clean hydrogen in an electrolyzer.”