Lithium-ion batteries have emerged as the leading technology in energy storage for applications like consumer electronics, electric vehicles, and grid-scale storage. Yet, their applicability for large-scale grid energy storage is questioned due to safety issues linked to volatile materials, particularly the electrolytes, used in their construction.
In response to this concern, researchers at RMIT University have developed a solution by replacing the hazardous chemical electrolyte in lithium-ion batteries with water.
A “greener, safer” alternative to lead-acid batteries
These recyclable “water batteries” eliminate the risk of fire or explosion, potentially replacing conventional lead-acid batteries and providing a safer and more environmentally friendly alternative.
The water battery, referred to as an “aqueous metal-ion battery,” functions similarly to conventional lead-acid batteries but with added benefits. It can be readily recycled without the risk of chemical pollution or the necessity for specialized equipment and disposal facilities, making it a more environmentally friendly option. Moreover, water batteries boast cost advantages. Their simplified manufacturing processes and lower material costs mean they can be produced at a fraction of the expense of lithium-ion batteries, making them approximately one-third cheaper.
Versatile Prototypes Showcase Robust Performance
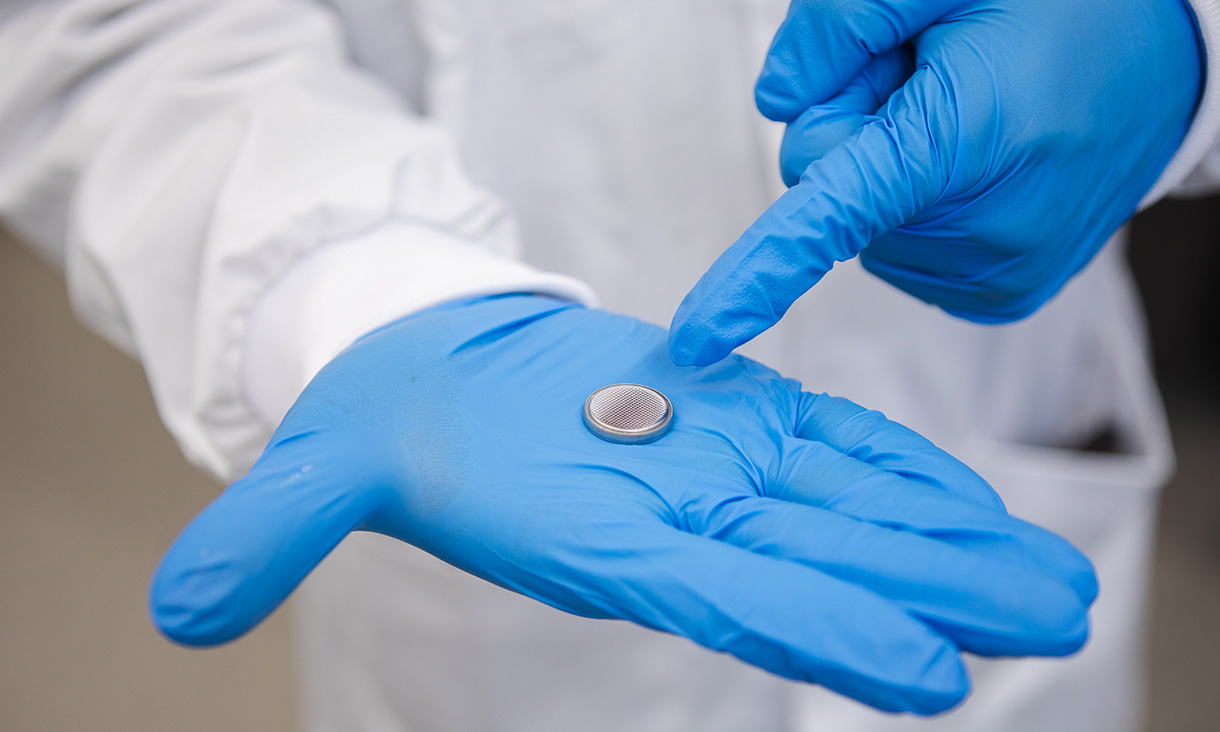
The research team has successfully prototyped water batteries in various formats, including coin-cell-type devices (commonly found in small clocks), battery packs reminiscent of old Nokia-style mobile phones, and AA-style cylinder batteries. These prototypes have demonstrated impressive performance, achieving 500 or more charge cycles while maintaining 80% of their capacity after 700 cycles.
Researchers anticipate that with further technological advancements, these water batteries could eventually pose a competitive challenge to lithium-ion batteries.