In a big breakthrough, researchers at Rice University have discovered a way to recycle carbon dioxide to produce pure liquid fuel solutions using electricity.
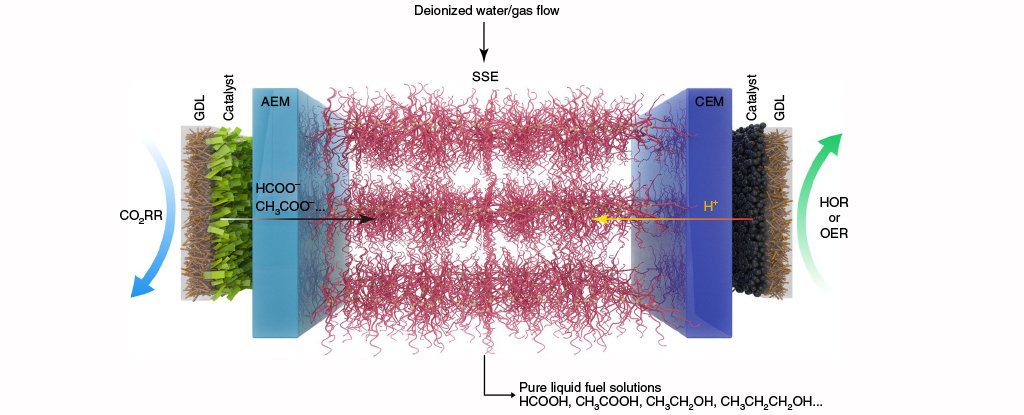
Researchers developed a catalytic reactor to convert carbon dioxide into high concentrations of pure formic acid, which can be used as a liquid fuel.
Researcher Haotian Wang, a chemical and biomolecular engineer at Rice, said, “Formic acid is an energy carrier. It’s a fuel-cell fuel that can generate electricity and emit carbon dioxide — which you can grab and recycle again.”
Researchers built an electro-catalysis reactor for recycling CO2 in a cost-effective and energy-efficient way
Using present technologies, conversion of CO2 into pure formic acid involves chain of costly purification procedures. However, Rice University researchers made use of two-dimensional bismuth catalyst and a solid-state electrolyte. Bismuth being a very heavy atom, with low mobility, has great effect of stabilizing the catalyst. Best part is fuel generated by the new device is pure and there is no need to purify it.
“Currently, people produce catalysts on the milligram or gram scales,” researcher Dr. Chuan Xia said. “We developed a way to produce them at the kilogram scale. That will make our process easier to scale up for industry.”
“Currently, people produce catalysts on the milligram or gram scales. We developed a way to produce them at the kilogram scale. That will make our process easier to scale up for industry.”
He added, “Electrocatalytic CO2 reduction reaction (CO2RR) to form valuable liquid fuels using renewable energy is a potential strategy to achieve a carbon-neutral energy cycle,”